

Huntingtin-associated protein 1 (Hap1), originally identified as a neuronal protein that interacts with the Huntington disease (HD) protein, huntingtin (htt), is critical for postnatal development, as Hap1-KO mice often die before P3 due to inhibited feeding behavior ( 2, 3). Because aberrant postnatal brain maturation can be caused by multiple mechanisms and leads to a variety of neurological and psychiatric disorders such as schizophrenia ( 1), it is important to understand the role of postnatal neurogenesis and find out important molecules that regulate this process. Unlike embryonic neurogenesis, which is largely controlled by transcription factors, and adult neurogenesis, which is restricted to a few brain regions, postnatal neurogenesis is critical for the maturation of neuronal connections in the central nervous system and is profoundly influenced by environmental factors after birth.
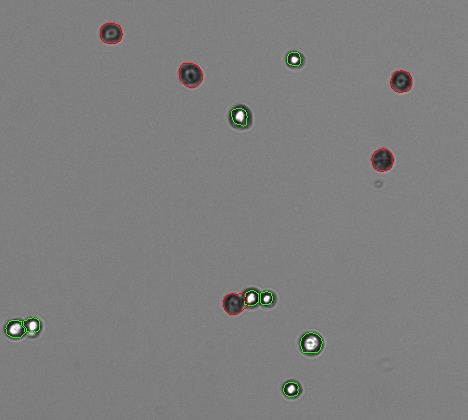
Neurogenesis is the process by which neurons are generated from neural stem and progenitor cells. Our findings indicate that intracellular sorting of neurotrophin receptors is critical for postnatal neurogenesis and could provide a therapeutic target for defective postnatal neurogenesis. HAP1 stabilized the association of TRKB with the intracellular sorting protein sortilin, prevented TRKB degradation, and promoted its anterograde transport. In the absence of HAP1, postnatal hypothalamic neurons exhibited reduced receptor tropomyosin-related kinase B (TRKB) levels and decreased survival. Neurogenesis, but not gliogenesis, was affected in HAP1-null neurospheres and mouse brain.
We found that HAP1 reduction selectively affects survival and growth of postnatal mice, but not adults. Here, we used tamoxifen-induced (TM-induced) Cre recombination to deplete HAP1 in mice at different ages. Huntingtin-associated protein 1 (HAP1) participates in intracellular trafficking in neurons, and its absence leads to postnatal death in mice. Defective neurogenesis in the postnatal brain can lead to many neurological and psychiatric disorders, yet the mechanism behind postnatal neurogenesis remains to be investigated.


 0 kommentar(er)
0 kommentar(er)
